Sulfur dioxide (chemical formula: SO2), also known as sulfite anhydride, is the most common sulfur oxide, the main component of sulfuric acid feed gas. Sulfur dioxide is a colorless gas with a strong pungent odor and is one of the main pollutants in the atmosphere. This gas is emitted when a volcano erupts, and sulfur dioxide is also produced in many industrial processes. Since coal and petroleum usually contain sulfur compounds, sulfur dioxide is formed during combustion. When sulfur dioxide is dissolved in water, sulfurous acid (the main component of acid rain) is formed. If SO2 is further oxidized in the presence of a catalyst such as nitrogen dioxide, sulfuric acid (H2SO4) is formed, which should be handled with caution when it comes into contact with the skin.
basic structure
SO2 is a curved molecule whose symmetry point group is C2v. The sulfur atom has an oxidation state of +4 and a formal charge of 0, surrounded by five electron pairs, and thus can be described as a supervalent molecule. From the point of view of molecular orbital theory, it can be considered that most of these valence electrons participate in the formation of SO bonds.
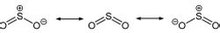
Sulfur dioxide structure
The SO bond length in SO2 (143.1 pm) is shorter than the SO bond length in sulfur monoxide (148.1 pm), while the OO bond length in O3 (127.8 pm) is longer than the OO bond in oxygen O2 (120.7 pm). long. The average bond energy of SO2 (548 kJ mol) is greater than the average bond energy of SO (524 kJ mol), while the average bond energy of O3 (297 kJ mol) is less than the average bond energy of O2 (490 kJ mol). This evidence led chemists to conclude that the SO bond in sulfur dioxide has a bond level of at least 2, unlike the OO bond in ozone, where the bond of the OO bond in the ozone is 1.5.
Molecular structure and polarity: V-shaped molecules, polar molecules.
production method
Industrial preparation
Sulfur dioxide (SO2)
Industrial preparation
The method for preparing sulfur dioxide is: incinerating sulfur; roasting pyrite or non-ferrous sulfide ore; incinating a gas containing hydrogen sulfide; calcining gypsum or phosphogypsum; heating and decomposing waste sulfuric acid or ferrous sulfate; and burning smoke from sulfur-containing fuel Recycling in the gas (see sulfuric acid feed gas).
Pure sulfur dioxide gas is usually produced first in the production of liquid sulfur dioxide and then liquefied by compression or freezing. Important industrial production methods are:
1 Hanesh-Hilot method. This method was founded in 1884, using water as an absorbent. The solution after absorbing sulfur dioxide is desorbed by steam. The desorbed gas is condensed, dried and liquefied. A pressurized water absorption method has been developed.
2 ammonia-sulfuric acid method. This method is commonly used in the recovery of tail gas sulfur dioxide in a contact sulfuric acid plant. Ammonia water is used as the original absorbent, and the absorption liquid is decomposed with sulfuric acid to obtain pure sulfur dioxide gas.
3 solution absorption method. A low concentration of sulfur dioxide gas is absorbed by an inorganic or organic solution, and then the absorption liquid is heated and regenerated to obtain pure sulfur dioxide. The main absorbents are solutions of sodium carbonate, sodium citrate, basic aluminum sulfate, organic amines and the like.
4 direct condensation method. The sulphur dioxide-containing gas is partially condensed and separated by a freezing method to directly prepare liquid sulphur dioxide, and the uncondensed sulphur dioxide is returned to the sulphuric acid production system.
5 sulfur trioxide-sulfur method. The liquid sulfur is reacted with sulfur trioxide in a reactor to obtain a pure sulfur dioxide gas.
Laboratory preparation
The laboratory usually uses sodium sulfite to react with concentrated sulfuric acid to prepare sulfur dioxide.
Na2SO3+H2SO4=Na2SO4+SO2(g)+H2O
Or heating with copper and concentrated sulfuric acid
Cu+2H2SO4=△=CuSO4+SO2(g)+2H2O
Exhaust gas treatment: pass sodium hydroxide solution
2NaOH+SO2=Na2SO3+H2O
Other methods
Sulfur dioxide can be generated under sulfur burning conditions
S(s)+O2(g) → SO2(g)
Hydrogen sulfide can be burned to form sulfur dioxide
2H2S(g) +3O2(g) → 2H2O(g) +2SO2(g)
Heating pyrite, sphalerite, mercury sulfide, can produce sulfur dioxide
4FeS2(s) +11O2(g) → 2Fe2O3(s) +8SO2(g)
2ZnS(s) +3O2(g) → 2ZnO(s) +2SO2(g)
HgS(s) +O2(g) → Hg(g) +SO2(g)

Inkjet Receptive Coating, Anticorrosion Pigments, Matting Agent